ES
Executive Summary
Land degradation affects people and ecosystems throughout the planet and is both affected by climate change and contributes to it. In this report, land degradation is defined as a negative trend in land condition, caused by direct or indirect human-induced processes including anthropogenic climate change, expressed as long-term reduction or loss of at least one of the following: biological productivity, ecological integrity, or value to humans. Forest degradation is land degradation that occurs in forest land. Deforestation is the conversion of forest to non-forest land and can result in land degradation. {4.1.3}
Land degradation adversely affects people’s livelihoods (very high confidence) and occurs over a quarter of the Earth’s ice-free land area (medium confidence). The majority of the 1.3 to 3.2 billion affected people (low confidence) are living in poverty in developing countries (medium confidence).
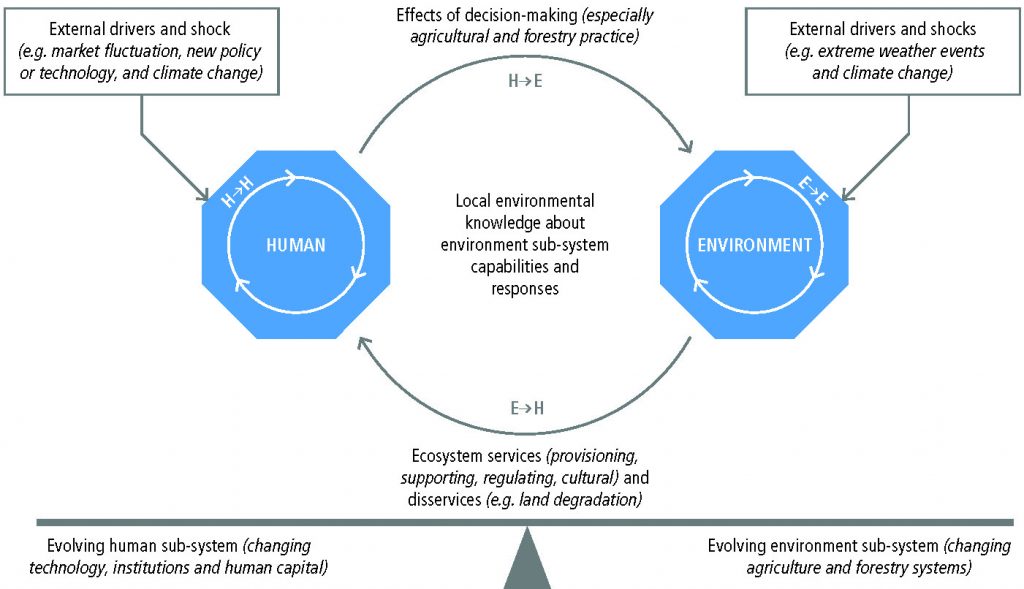
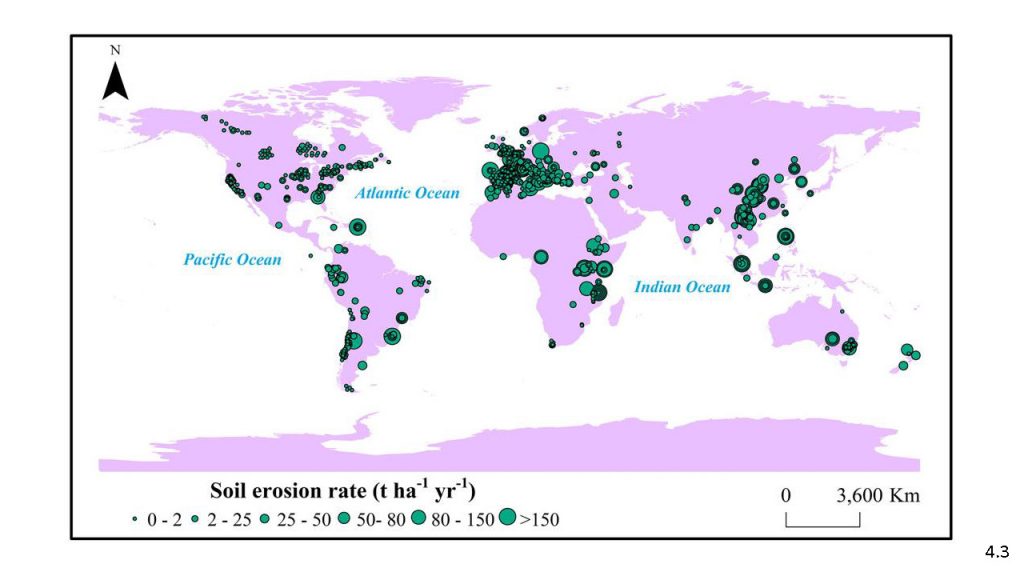
Land-use changes and unsustainable land management are direct human causes of land degradation (very high confidence), with agriculture being a dominant sector driving degradation (very high confidence). Soil loss from conventionally tilled land exceeds the rate of soil formation by >2 orders of magnitude (medium confidence). Land degradation affects humans in multiple ways, interacting with social, political, cultural and economic aspects, including markets, technology, inequality and demographic change (very high confidence). Land degradation impacts extend beyond the land surface itself, affecting marine and freshwater systems, as well as people and ecosystems far away from the local sites of degradation (very high confidence). {4.1.6, 4.2.1, 4.2.3, 4.3, 4.6.1, 4.7, Table 4.1}
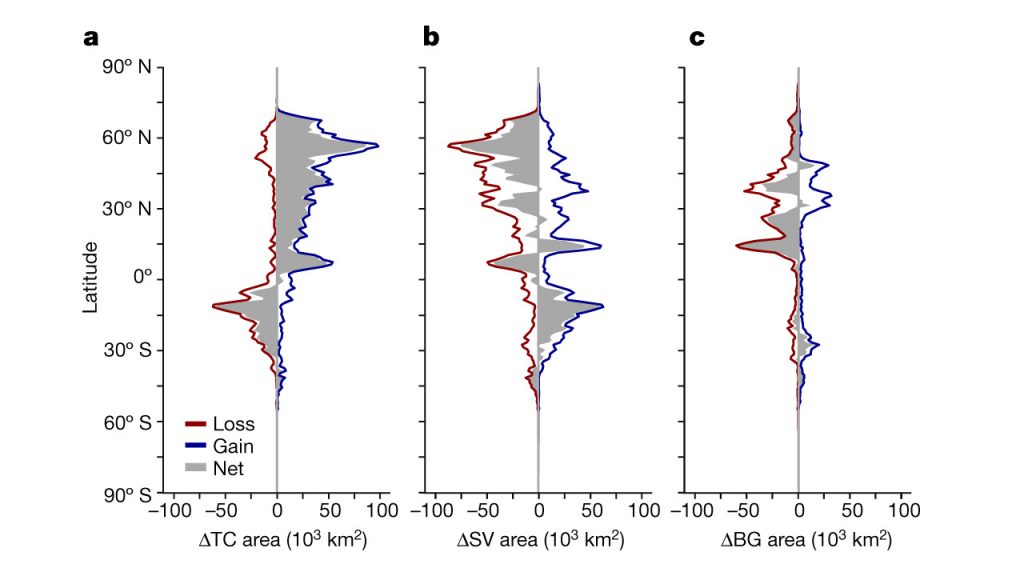
Climate change exacerbates the rate and magnitude of several ongoing land degradation processes and introduces new degradation patterns (high confidence). Human-induced global warming has already caused observed changes in two drivers of land degradation: increased frequency, intensity and/or amount of heavy precipitation (medium confidence); and increased heat stress (high confidence). In some areas sea level rise has exacerbated coastal erosion (medium confidence). Global warming beyond present day will further exacerbate ongoing land degradation processes through increasing floods (medium confidence), drought frequency and severity (medium confidence), intensified cyclones (medium confidence), and sea level rise (very high confidence), with outcomes being modulated by land management (very high confidence). Permafrost thawing due to warming (high confidence), and coastal erosion due to sea level rise and impacts of changing storm paths (low confidence), are examples of land degradation affecting places where it has not typically been a problem. Erosion of coastal areas because of sea level rise will increase worldwide (high confidence). In cyclone prone areas, the combination of sea level rise and more intense cyclones will cause land degradation with serious consequences for people and livelihoods (very high confidence). {4.2.1, 4.2.2, 4.2.3, 4.4.1, 4.4.2, 4.9.6, Table 4.1}
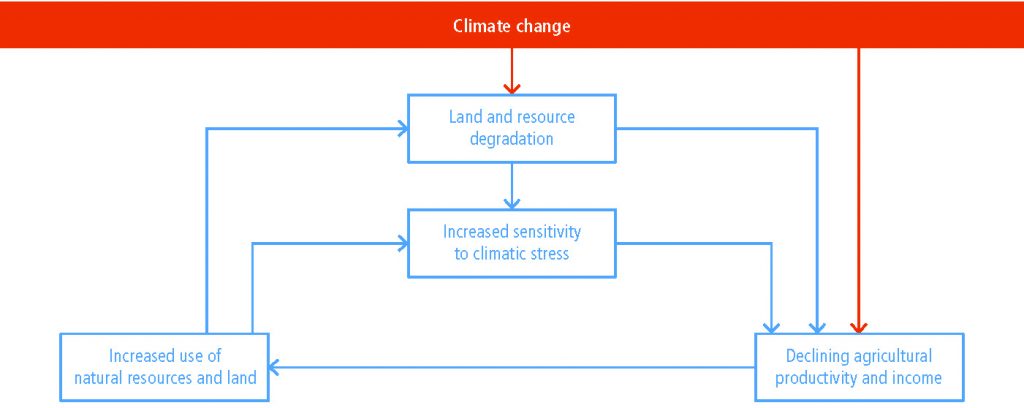
Land degradation and climate change, both individually and in combination, have profound implications for natural resource-based livelihood systems and societal groups (high confidence)
The number of people whose livelihood depends on degraded lands has been estimated to be about 1.5 billion worldwide (very low confidence). People in degraded areas who directly depend on natural resources for subsistence, food security and income, including women and youth with limited adaptation options, are especially vulnerable to land degradation and climate change (high confidence). Land degradation reduces land productivity and increases the workload of managing the land, affecting women disproportionally in some regions. Land degradation and climate change act as threat multipliers for already precarious livelihoods (very high confidence), leaving them highly sensitive to extreme climatic events, with consequences such as poverty and food insecurity (high confidence) and, in some cases, migration, conflict and loss of cultural heritage (low confidence). Changes in vegetation cover and distribution due to climate change increase the risk of land degradation in some areas (medium confidence). Climate change will have detrimental effects on livelihoods, habitats and infrastructure through increased rates of land degradation (high confidence) and from new degradation patterns (low evidence, high agreement). {4.1.6, 4.2.1, 4.7}
Land degradation is a driver of climate change through emission of greenhouse gases (GHGs) and reduced rates of carbon uptake (very high confidence). Since 1990, globally the forest area has decreased by 3% (low confidence) with net decreases in the tropics and net increases outside the tropics (high confidence). Lower carbon density in re-growing forests, compared to carbon stocks before deforestation, results in net emissions from land-use change (very high confidence). Forest management that reduces carbon stocks of forest land also leads to emissions, but global estimates of these emissions are uncertain. Cropland soils have lost 20–60% of their organic carbon content prior to cultivation, and soils under conventional agriculture continue to be a source of GHGs (medium confidence). Of the land degradation processes, deforestation, increasing wildfires, degradation of peat soils, and permafrost thawing contribute most to climate change through the release of GHGs and the reduction in land carbon sinks following deforestation (high confidence). Agricultural practices also emit non-CO2 GHGs from soils and these emissions are exacerbated by climate change (medium confidence). Conversion of primary to managed forests, illegal logging and unsustainable forest management result in GHG emissions (very high confidence) and can have additional physical effects on the regional climate including those arising from albedo shifts (medium confidence). These interactions call for more integrative climate impact assessments. {4.2.2, 4.3, 4.5.4, 4.6}
Large-scale implementation of dedicated biomass production for bioenergy increases competition for land with potentially serious consequences for food security and land degradation (high confidence). Increasing the extent and intensity of biomass production, for example, through fertiliser additions, irrigation or monoculture energy plantations, can result in local land degradation. Poorly implemented intensification of land management contributes to land degradation (e.g., salinisation from irrigation) and disrupted livelihoods (high confidence). In areas where afforestation and reforestation occur on previously degraded lands, opportunities exist to restore and rehabilitate lands with potentially significant co-benefits (high confidence) that depend on whether restoration involves natural or plantation forests. The total area of degraded lands has been estimated at 10–60 Mkm2 (very low confidence). The extent of degraded and marginal lands suitable for dedicated biomass production is highly uncertain and cannot be established without due consideration of current land use and land tenure. Increasing the area of dedicated energy crops can lead to land degradation elsewhere through indirect land-use change (medium confidence). Impacts of energy crops can be reduced through strategic integration with agricultural and forestry systems (high confidence) but the total quantity of biomass that can be produced through synergistic production systems is unknown. {4.1.6, 4.4.2, 4.5, 4.7.1, 4.8.1, 4.8.3, 4.8.4, 4.9.3}
Reducing unsustainable use of traditional biomass reduces land degradation and emissions of CO2 while providing social and economic co-benefits (very high confidence). Traditional biomass in the form of fuelwood, charcoal and agricultural residues remains a primary source of energy for more than one-third of the global population, leading to unsustainable use of biomass resources and forest degradation and contributing around 2% of global GHG emissions (low confidence). Enhanced forest protection, improved forest and agricultural management, fuel-switching and adoption of efficient cooking and heating appliances can promote more sustainable biomass use and reduce land degradation, with co-benefits of reduced GHG emissions, improved human health, and reduced workload especially for women and youth (very high confidence). {4.1.6, 4.5.4}
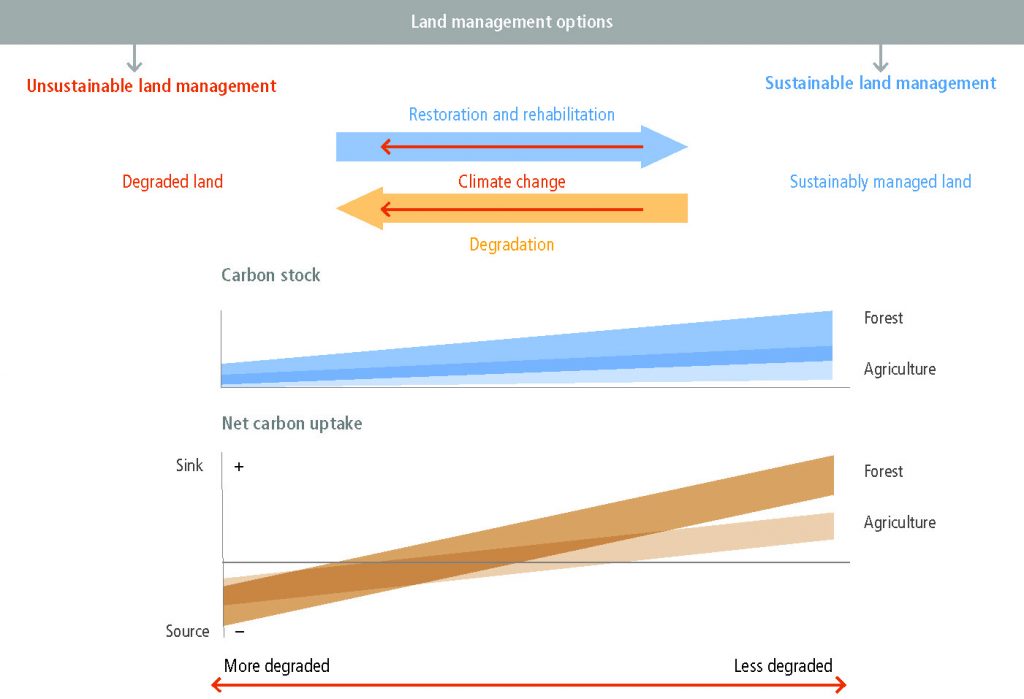
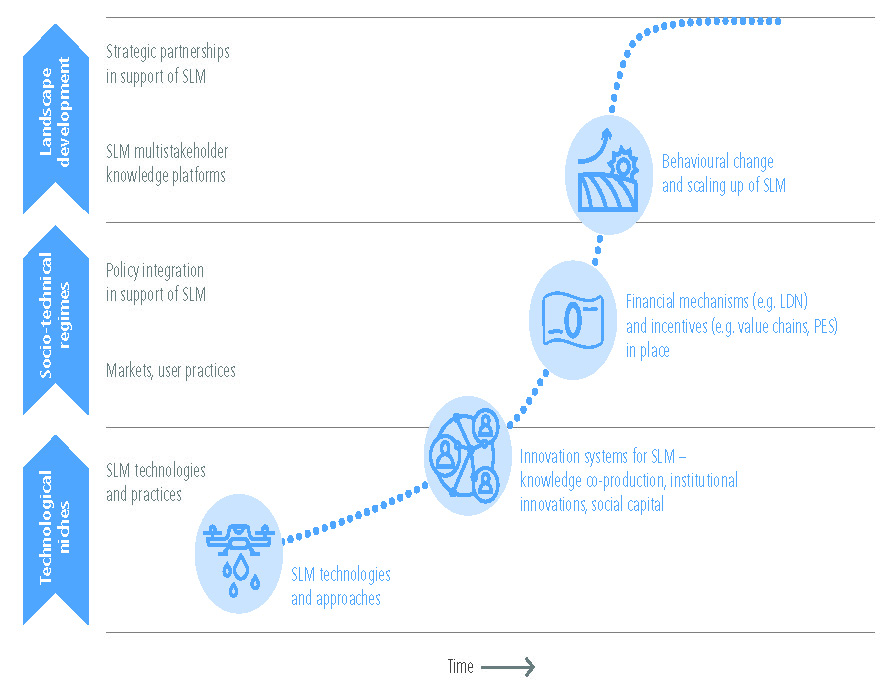
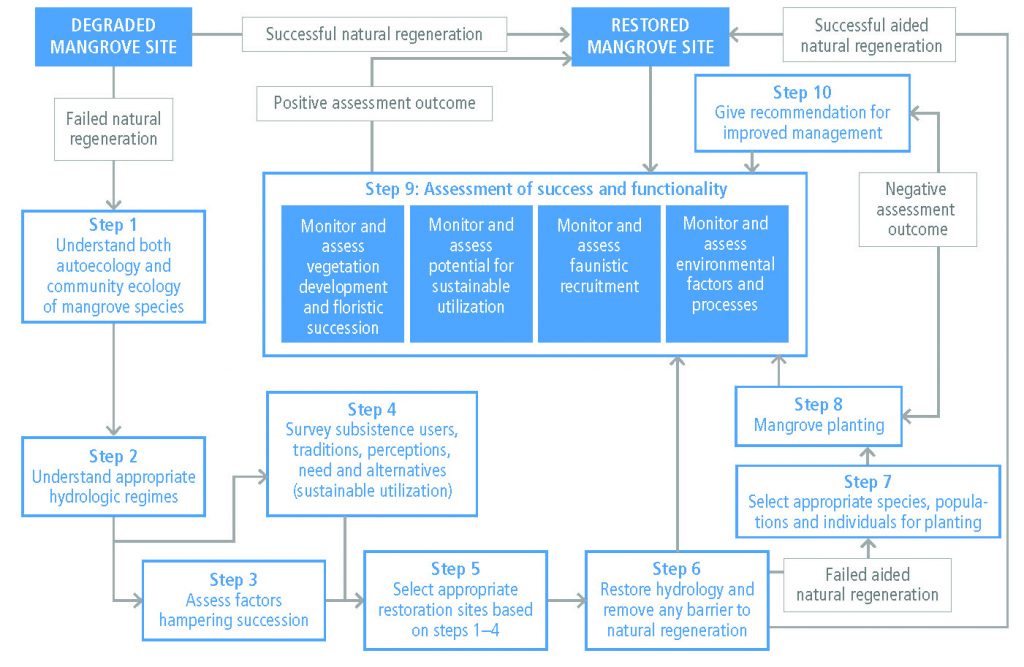
Land degradation can be avoided, reduced or reversed by implementing sustainable land management, restoration and rehabilitation practices that simultaneously provide many co-benefits, including adaptation to and mitigation of climate change (high confidence). Sustainable land management involves a comprehensive array of technologies and enabling conditions, which have proven to address land degradation at multiple landscape scales, from local farms (very high confidence) to entire watersheds (medium confidence). Sustainable forest management can prevent deforestation, maintain and enhance carbon sinks and can contribute towards GHG emissions-reduction goals. Sustainable forest management generates socio-economic benefits, and provides fibre, timber and biomass to meet society’s growing needs. While sustainable forest management sustains high carbon sinks, the conversion from primary forests to sustainably managed forests can result in carbon emission during the transition and loss of biodiversity (high confidence). Conversely, in areas of degraded forests, sustainable forest management can increase carbon stocks and biodiversity (medium confidence). Carbon storage in long-lived wood products and reductions of emissions from use of wood products to substitute for emissions-intensive materials also contribute to mitigation objectives. {4.8, 4.9, Table 4.2}
Lack of action to address land degradation will increase emissions and reduce carbon sinks and is inconsistent with the emissions reductions required to limit global warming to 1.5°C or 2°C. (high confidence). Better management of soils can offset 5–20% of current global anthropogenic GHG emissions (medium confidence). Measures to avoid, reduce and reverse land degradation are available but economic, political, institutional, legal and socio-cultural barriers, including lack of access to resources and knowledge, restrict their uptake (very high confidence). Proven measures that facilitate implementation of practices that avoid, reduce, or reverse land degradation include tenure reform, tax incentives, payments for ecosystem services, participatory integrated land-use planning, farmer networks and rural advisory services. Delayed action increases the costs of addressing land degradation, and can lead to irreversible biophysical and human outcomes (high confidence). Early actions can generate both site-specific and immediate benefits to communities affected by land degradation, and contribute to long-term global benefits through climate change mitigation (high confidence). {4.1.5, 4.1.6, 4.7.1, 4.8, Table 4.2}
Even with adequate implementation of measures to avoid, reduce and reverse land degradation, there will be residual degradation in some situations (high confidence). Limits to adaptation are dynamic, site specific and determined through the interaction of biophysical changes with social and institutional conditions. Exceeding the limits of adaptation will trigger escalating losses or result in undesirable changes, such as forced migration, conflicts, or poverty. Examples of potential limits to adaptation due to climate-change-induced land degradation are coastal erosion (where land disappears, collapsing infrastructure and livelihoods due to thawing of permafrost), and extreme forms of soil erosion. {4.7, 4.8.5, 4.8.6, 4.9.6, 4.9.7, 4.9.8}
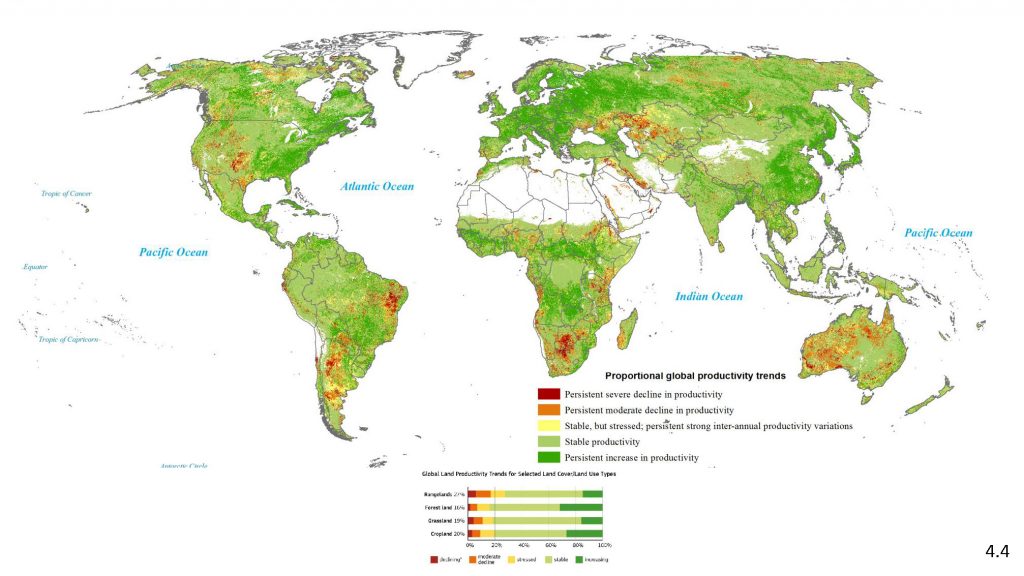
Land degradation is a serious and widespread problem, yet key uncertainties remain concerning its extent, severity, and linkages to climate change (very high confidence). Despite the difficulties of objectively measuring the extent and severity of land degradation, given its complex and value-based characteristics, land degradation represents – along with climate change – one of the biggest and most urgent challenges for humanity (very high confidence). The current global extent, severity and rates of land degradation are not well quantified. There is no single method by which land degradation can be measured objectively and consistently over large areas because it is such a complex and value-laden concept (very high confidence). However, many existing scientific and locally-based approaches, including the use of indigenous and local knowledge, can assess different aspects of land degradation or provide proxies. Remote sensing, corroborated by other data, can generate geographically explicit and globally consistent data that can be used as proxies over relevant time scales (several decades). Few studies have specifically addressed the impacts of proposed land-based negative emission technologies on land degradation. Much research has tried to understand how livelihoods and ecosystems are affected by a particular stressor – for example, drought, heat stress, or waterlogging. Important knowledge gaps remain in understanding how plants, habitats and ecosystems are affected by the cumulative and interacting impacts of several stressors, including potential new stressors resulting from large-scale implementation of negative emission technologies. {4.10}